|
|
Water Chemistry I: Dilutions & Solute Concentration
How can you test an unknown solution to determine its solute concentration? The method described below relies upon standard lab equipment, and while we used it to determine protein concentration, it has a wide variety of applications. Spectrophotometry can be used for determining the amount of many types of substances in solution, from living bacteria cells to DNA fragments.
|
|
Spectrophotometry and Protein Concentration
A spectrophotometer is a machine that measures light quantity. It can tell you how much light is passing through a solution (transmittance) or how much light is being absorbed by a solution (absorbance).
We started by mixing known amounts of a protein (albumin) with a dye indicator called Coomassie Blue. The dye starts out reddish brown, but turns blue when it reacts with protein. The more protein present, the darker blue the indicator turns. As the amount of protein in a solution increases, the color of the solution becomes darker. The darker colored the solution, the more light it absorbs. (Note the tubes across the title of this page -- they are the set of samples set up for this experiment).
If you graph light absorbance versus concentration for a series of solutions of known concentration, the line, or standard curve, which fits to your points can be used to figure out the concentrations of unknown solutions. You measure their absorbance, find that point on the standard curve, and then see which concentration matches up to it.
|
To Generate a Standard Curve
First, serially dilute a stock solution of known concentration. This gives you a set of tubes of varying but known concentrations.
Second,
add dye to the tubes and allow full color development.
Third, measure and graph absorbances versus solute concentration.
|
| |
Set Up and Dilution: Steps 1-2
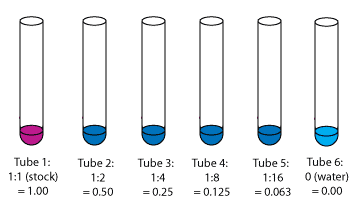
Starting with 2 ml of stock albumin solution, we did a dilution series (mouse over image to see dilutions) to get six tubes of varying albumin concentrations. |
|
Step 3: Dilution Factors of the Tubes
After dilution, each tube contained 1 ml of liquid. Our tube dilutions were: 1 (100% undiluted stock), 1/2 (50% concentration), 1/4 (25% of stock concentration), 1/8 (12.5%), 1/16 (6.25%), and 0 (pure water containing no albumin -- we used this as our "blank" tube). |
Calculating Albumin Concentrations
We started with a stock solution of 0.1 mg/ml albumin. This is a very low concentration, and is more informative if expressed in terms of micrograms per ml.
To convert our units from milligrams to micrograms, use the following formula. There are 1,000 milligrams (mg) in a gram, and 1,000,000 micrograms (ug) in a gram. If 1,000 mg = 1,000,000 ug, then 1,000,000/ 1,000 is the number of micrograms per milligram. That is 1,000 micrograms per 1 milligram. To determine how many micrograms of albumin were in each of our tubes, we multiply mg albumin by 1,000 ug/mg.
That gives us: 0.1 mg/ml * 1000 ug/mg = 100 ug/ml (the mg cancel out).
To determine amount of albumin in each tube, we now multiply the concentration of our original solution by the dilution factor of each tube. See table below.
After dilution, each tube contained 1 ml of liquid. Next, 2 ml of dye was added to each tube, followed by 7 ml of water. Thus, the final volume of each tube was 10 ml. Solution volume was a controlled variable in this experiment. We kept it the same for all tubes so we didn't have to worry that it would impact our results. Our tube of unknown concentration (Unknown A) was set up in the same way: 1 ml of unknown + 2 ml dye + 7 ml water. Once the color had had 30 minutes to develop, we were ready to measure absorbances on the spectrophotometer. Go to Water Chemistry II to see our standard curve.
(The change in volume to 10 ml further diluted the abumin, but because we added the same amount of liquid to each tube, their concentrations remained the same relative to each other. This let us treat the final tubes as if we did not add any further liquid after our serial dilution.)
|
TUBE # |
DILUTION FACTOR |
STOCK CONC. |
DILUTED CONCENTRATION |
1 |
1 (pure stock) |
100 ug/ml |
1 * 100 ug/ml = 100 ug/ml |
2 |
1/2 or 0.5 |
100 ug/ml |
0.5 * 100 ug/ml = 50 ug/ml |
3 |
1/4 or 0.25 |
100 ug/ml |
0.25 * 100 ug/ml = 25 ug/ml |
4 |
1/8 or 0.125 |
100 ug/ml |
0.125 * 100 ug/ml = 13 ug/ml |
5 |
1/16 or 0.063 |
100 ug/ml |
0.063 * 100 ug/ml = 6 ug/ml |
6 |
0 (pure water) |
100 ug/ml |
0 * 100 ug/ml = 0 ug/ml |
|
|
|
|