Undergraduate research in
the Russell Group involves the synthesis and characterization
of enediynes, compounds possessing triple bonds on
either side of a cis-double bond. The molecules prepared
in the Russell Research Group are intended to find
uses in both material and biological sciences. The
Russell Group provides opportunities for undergraduates
to study enediynes in one of the following areas:
|
Dehydroheteroarylannulenes
Bergman Cyclization
Dendrimers
|
Dehydroheteroarylannulenes
|
| |
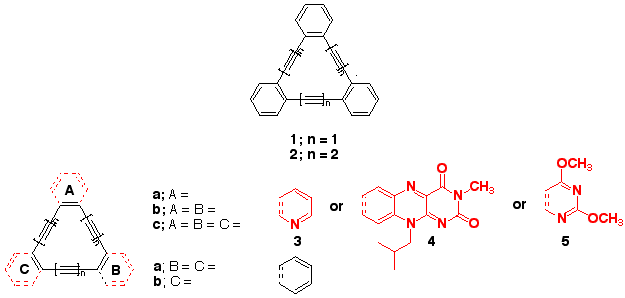
The dehydrobenzoannulenes
(DBAs) such as 1 and 2 are molecules that have benzene
rings separated by carbon-carbon
triple bonds. People are very interested in these types
of molecules because they have unusual chemical and physical
properties. These compounds are expected to be very important
in the design and manufacture of nanoelectronics, such
as molecule size wires, logic gates, and memory storage
devices.
The Russell group is interested in synthesizing new DBAs,
the so-called dehydroheteroarylannulenes (DHAs). In these
molecules one or more of the aromatic rings
(A-C in the
lower structure) is replaced with a heterocycle, such
as 3-5. Once prepared the molecules will be examined
in detail for their physical and chemical properties.
|
 |
| Current Researchers: Amber
Bisch, Jackie Bowman, Lindsey Easthon, Jason Ferayorni, Jim
Leslie, |
|
Katie
Martin and Mark
Seger |
|
Collaborators: Dr.
Haley (University of Oregon), Dr.
Bullen (NKU) |
| Funding : Research
Corporation, National
Science Foundation |
|
Bergman Cyclization
|
| |
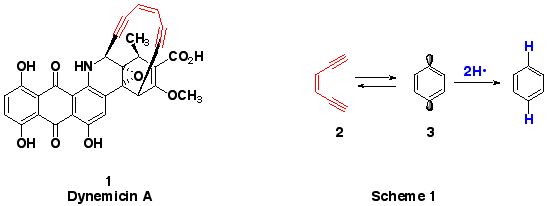
Among the most potent naturally occurring anticancer
agents is Dynemicin A (1). Dynemicin A is only one example
of a class of compounds known as the enediyne antibiotics.
Each of these molecules possesses an enediyne (red) which
undergoes a reaction that ultimately results in cancer
cell death. The reaction is called a BERGMAN CYCLIZATION
(Scheme 1). In a Bergman cyclization a cis 3-ene-1,5-diyne
(2) rearranges to a p-benzyne diradical (3). This highly
reactive diradical abstracts hydrogen atoms from wherever
it can get them. Biologically, the hydrogens come from
DNA. When these hydrogens are removed, the DNA becomes
damaged and the cancer cell dies.
|
|
| |
Our research interests lie
in trying to understand how the rate of the BERGMAN
CYCLIZATION reaction is changed
by various factors. Undergraduates in the Russell research
group have already helped demonstrate that when a double
bond is part of an aromatic ring with an electron withdrawing
group, the reaction goes faster. Using the compounds
below undergraduates will study a number of other
electronic effects on the
rate of BERGMAN CYCLIZATION.
|
Figure for Bergman goes
here |
| Current Researchers: Jim
Leslie, Jason
Ferayorni |
| Collaborators: Dr.
Parish (University of Richmond) |
| Funding : National
Science Foundation |
|
| |
Dendrimers |
| |
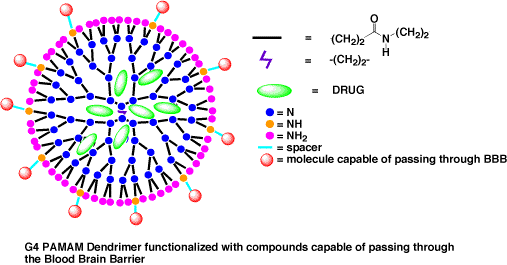
Dendrimers are large molecules
with chemically well defined structures. These compounds
can easily modified for a number of uses. Because
of their size and shape dendrimers are capable of carrying
drugs within them that can
later be released(see figure
below). The goal of our research is to synthesize
dendrimers that are capable of transporting drugs across
the Blood Brain Barrier (BBB). The BBB is a specialized
membrane that only allows certain molecules across.
Our strategy is to attach molecules known to cross
the BBB to the surface of a dendrimer. Such molecules
include GM1 ganglioside, 7-nitroindazole, docosahexenoic
acid, and 3-nitropropanoic acid. We will then attach
a dye to the dendrimer before passing on the compounds
to biologists to determine if the new molecules can
cross the BBB.
|
|
| Current Researchers: Matt
Lauer, Areej Saqr |
| Collaborators: Dr.
Martines (NKU) |
| Funding : CINSAM |
| |
| |
| |
| |
| |
| |
| |