|
|
Lipids
Fats, oils, and waxes are all examples of lipids. There are lots of lipids, but they all share the trait of being at least partially hydrophobic (meaning they won't mix with water).
Water molecules are polar because they have positive and negative ends, rather like little magnets. Most lipids are non-polar (having no charged areas) or only slightly polar, with a very few charged areas. Water mixes with hydrophilic (water-loving) compounds by sticking to their charged groups. Since lipids lack charged groups, the water molecules have nothing to stick to and don't mix with them. (Think of trying to pick up a glass marble with a magnet).
Lipids can be placed in three major groups: triglycerides (fats & oils), phospholipids (making up cell membranes), and steroids (many hormones).
|
|
Testing the Polarity of Lipids
We can use chromatography to separate out mixtures of chemicals and tell us something about their properties. Drops of unknowns are blotted near the bottom of a chromatography strip. The base of the strip is set in a solvent, and the solvent soaks its way up the strip like water moves up a sponge. The solvent also takes some of the unknowns along with it. Chemicals with a polarity similar to the solvent will be dissolved by it and move along the solvent front. Small molecules will move along faster than large ones. By looking at how far various chemicals move along the strip, you can get a feel for how polar they are and sometimes also how large they are.
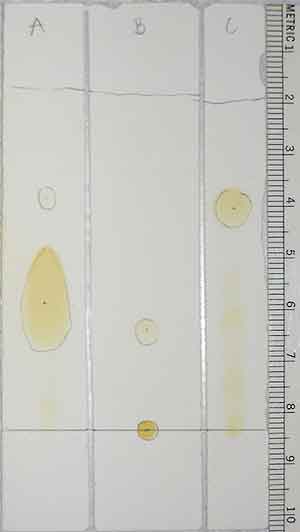
In this case, we placed three unknowns
on a chromatography strip: a phospholipid, a triglyceride, and a fatty acid. Our goal is to sort out which is which based on their properties. Our solvent is non-polar, and so should mix well with other non-polar substances and carry them along with it. We know that the more polar a compound, the tighter it should stick to the silica of the chromatography strip. Phospholipids shouldn't move at all from where they are placed on the strip, whereas triglycerides are non-polar and shouldn't stick at all to the strip. Fatty acids should fall somewhere in between.
|
 |
Rf Values
Rf A =
2.4 cm/ 6.5 cm
= 0.4
Rf B =
0 cm/ 6.5 cm
= 0.0
Rf C =
4.3 cm/ 6.5 cm
= 0.7
Note: Measurements are taken from the center of the darkest blotch in each lane.
|
 |
Calculations: Rf Values
Rf values are a numerical way of comparing the movement of various substances along a chromatography strip. The Rf is equal to the total distance a substance traveled (measure from the center of the blotch) divided by the total distance the solvent front moved. A substance that didn't move at all would have an Rf of zero, while a substance that moved right with the solvent front would have an Rf of one.
Distances can be calculated from the photo above by subtracting the final point to which something traveled from the point at which it started. For example, samples were blotted on at the 8.3 cm mark and the solvent stopped at the 1.8 cm mark. The solvent thus traveled 6.5 cm (or 8.3 cm - 1.8 cm). |
|
Class Chromatography Results
Based on class results, C moved fastest, A next fastest, and B didn't move (though you can see some smudging in all lanes from impurities). Thus, C should be non-polar like our solvent, A slightly polar, and B so polar that it couldn't be moved off the silica.
Triglycerides are non-polar, and should dissolve immediately in our non-polar solvent. Thus, C seems like a good candidate for our triglyceride sample. Phospholipids should adhere to the silica, which seems to match up with lane B. Fatty acids are slightly polar, which is the best match for lane A.
Can you see how the amount of polarity a substance has correlates with how fast it moves up the chromatography strip?
|
|
|
|