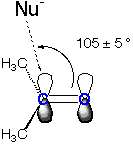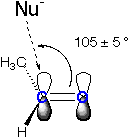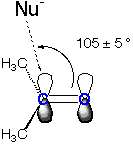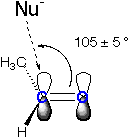The Burgi-Dunnitz Angle:
The Burgi-Dunitz angle describes the approach of a nucleophile (Nu) to an electrophilic carbon that is either part of a carbonyl or an enolate. It is defined as the Nu-C=0 angle for carbonyl electrophiles (ketones, aldehydes, etc) and the Nu-C=C angle for enolates and related electrophiles.
Burgi, Dunitz, Lehn, Wipff Tetrahedron,1974,30, 1563.
You can use the jmol plugin to measure the angles in the 3-D moleucles below. From the jmol menu choose Measurement: Click for angle measurement and you can select the atoms to determine the angles.
Symmetrical Ketone:
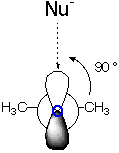
Addition of OH- to Acetone
| The Burgi-Dunitz angle
| End-on View
| 3-D Model
|
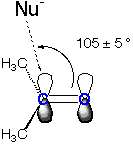
| 
|
|
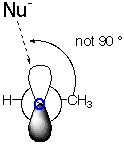
Aldehyde or Unymmetrical Ketone: Addition of OH- to Acetaldehyde
| The Burgi-Dunitz angle
| End-on View
| 3-D Model
|
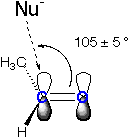
| 
|
|
return to Dr Russell's home page | tutorials
Updated March 5, 2013. KC Russell, Northern Kentucky University
© 2001-2013 KC Russell