Acid/Base Equilibria Problem 1
Tutorial to help us answer problem 1.
To find the equilibrium concentration of H+, we can use the following equation,
![[H+}{HC2O4^-]/[H2C2O4] = 5.6 x 10^-2.](images/acidbaseex1Teqn1.gif)
We ignore the H+ contribution from the loss of the second proton because
Ka2 << Ka1.
Let x denote [H+] at equilibrium. Then we have,
![[H+][HC2O4-]/[H2C2O4] = x^2/(0.10 - x) = 5.6 x 10^-2.](images/acidbaseex1Teqn2.gif)
NOTE: If x is small compared to 0.10 (i.e. 0.10 − x = 0.10 to two significant figures), then we can neglect x. We can make this simplification when the value of [acid]/ Ka is large (i.e. greater than 100). We cannot make the simplification in this example, and we proceed. |
We can now write the above equation as a quadratic equation by multiplying both sides by the denominator of the left hand side,
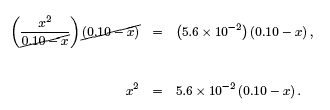
Expanding the above equation and bringing all terms to one side gives,
x2 + (5.6 × 10-2)x − (5.6 × 10-3) = 0.
The quadratic equation, ax2 + bx + c = 0, can be solved using the quadratic formula,
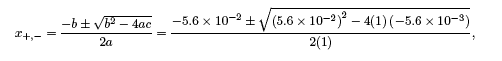
yielding the solutions,
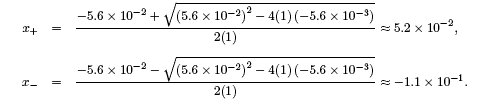
Since x represents the concentration of H+, the only answer that make sense is x ≈ 5.2 x 10-2 M.
