The Diels - Alder Reaction
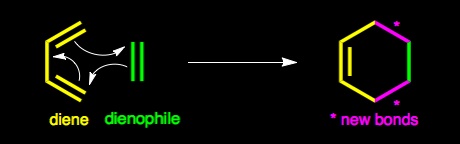
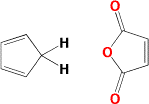
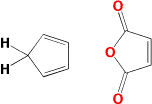
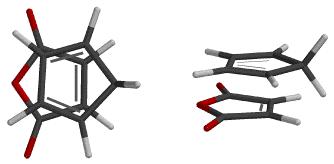
The Diels - Alder reaction is named after Otto Diels and Kurt Alder. In 1950 they were awarded the Nobel Prize for their work on this reaction. The Diels - Alder reaction is a cycloaddtion reaction between a two conjugated double bonds (a diene) and a carbon carbon double bond or triple bond (dienophile). The reaction is very important because two new carbon-carbon bonds and a cyclohexene ring are formed in a single step. The recation mechanism is pericyclic or concerted. In other words, the reaction is a single step process with no intermediates.