
Our atmosphere, so clear, so blue, is occasionally punctuated by clouds. Last night a huge thunderstorm ripped through my area, and the beautiful atmosphere gave way to a wild and tempestuous sky.
If not for our gaseous atmosphere, the weather would be very boring: "no weather again, today; tomorrow, no weather either."
It was the French mathematician and physicist Joseph Fourier who observed, in about 1824, that our planet would be uninhabitable if not for the atmosphere.
Fourier didn't know that the atmosphere is made up of about 78% nitrogen, 21% oxygen, and 1% argon. That doesn't leave much room for anything else, does it? I rounded everything to the nearest percent, and if we total it up it's 100%! But there's about .04% carbon dioxide by mass (or about 400 parts per million). But Fourier did know that it is the blanket of gasses that make up our atmosphere that keep in the sun's warmth; that keep some of that heat from radiating away, back to space.
You put that all together into a soup and you come up with a pleasant annual global temperature of around 14 degrees C, or 57 degrees F. But another scientist of the 19th century,
| John Tyndall, discovered something interesting about the gases in the atmosphere that are based on carbon: they retain infrared energy. They are the culprits that won't let all that heat escape back to space. |  |
Here's a picture of his apparatus, which demonstrated the effect:
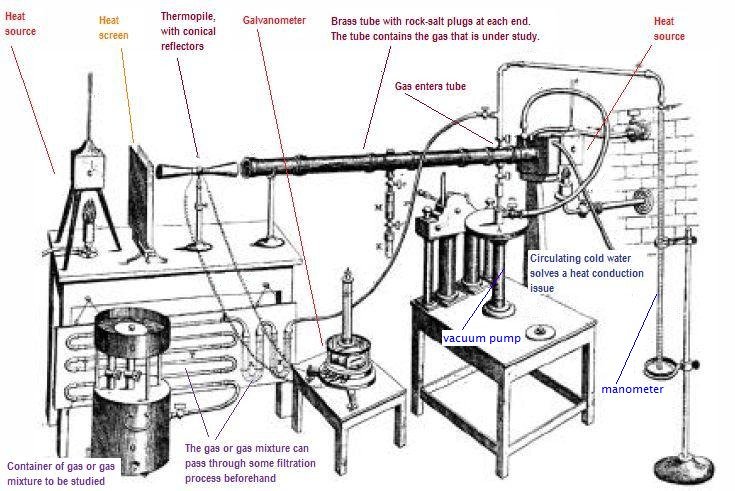
In Tyndall's paper, he describes how the galvanometer was plagued by misbehaviors caused by the green coloring of a piece of silk. It is so quaint to read these old scientific papers, because the scientists just talk to you. They are very conversational. Modern day science writers just don't cut it (or rather they cut it too much: they're too cut and dried).
Now Joseph Fourier knew that energy comes in different frequencies (back to his Fourier series): visible light is of a higher frequency (and shorter wavelength) than infrared "light", which is longer wavelength, and lower frequency. The product of the wavelength and frequency is the speed of light, so those two are "inversely related" to each other: when wavelength is large, frequency must be short (and vice versa)
In the end, Tyndall's experiments mean something truly astounding, however: that our atmosphere's tiny constituent, CO2, is blocking the infrared heat produced by the Earth in response to the sun's rays from re-radiating out to space. So that heat stays near the Earth and heats it up. Let me emphasize that we've known about this for about 150 years: carbon dioxide in our atmosphere will block infrared energy radiated by the Earth, retaining it, and warming the Earth. Now: as we have increased the amount of carbon dioxide in the atmosphere, so have we increased the temperature of the Earth (to first order).
But it's not only carbon dioxide that traps radiation. Here's Canadian Gilbert Plass describing the situation as he understood it in 1952: "The three most abundant gases in our atmosphere are oxygen, nitrogen, and argon. However, none of these three gases absorb appreciably in the relevant spectral region in the infrared. If these were the only gases in our atmosphere, our climate would be considerably colder than it is today. The heat radiated from the surface of the Earth would not be stopped in its passage out to space with the result that the Earth's surface would cool rapidly.
"Fortunately for us, three other gases occur in our atmosphere in relatively minute quantities: carbon dioxide, water vapor, and ozone. Unlike the more abundant gases, all three of these rarer gases absorb strongly over at least a portion of the infrared spectrum. The concentration of carbon dioxide in the atmosphere is about 0.03 per cent by volume, it is fairly uniformly mixed as high as accurate measurements have been made. Water vapor and ozone also exist in very small concentrations in the atmosphere, but the exact amount that is present varies with time and place.

"The infrared absorption properties of carbon dioxide, water vapor, and ozone determine our climate to a large extent. Their action has often been compared to that of a greenhouse. There the rays of the sun bring the heat energy in through the transparent glass. However, the outgoing heat energy from the plants and other objects in the greenhouse is in the infrared where glass is largely opaque. The heat energy is fairly effectively trapped inside the greenhouse and the temperature is considerably warmer than outside."
Blackbodies
"Any body at any temperature above absolute zero will radiate to some extent, the intensity and frequency distribution of the radiation depending on the detailed structure of the body. To begin analyzing heat radiation, we need to be specific about the body doing the radiating: the simplest possible case is an idealized body which is a perfect absorber, and therefore also (from the above argument) a perfect emitter. For obvious reasons, this is called a black body." (source)
So there are these spectra for each molecule, which indicate where each molecule is absorbing which frequency of radiation (and hence preventing its transmission out into space). In consequence this warms the Earth (the "blackbody"), which then begins to transmit energy at a different radiation profile. It shifts its emissions wavelengths, and, these new frequencies can leak out and lead to an energy balance (an equilibrium -- incoming balancing outgoing radiation).
| Here's the picture for Earth: |

|
Well, in the end what's important is that Fourier became the Newton of Heat. That's what I want you to remember. [Okay, so I'm lying!] The thing to remember is that a blanket of gas is keeping us warm, and it's doing so by intercepting the Earth's radiant energy and trapping it near the Earth -- forcing the Earth to get hotter. As it gets hotter, it radiates in different frequencies, until enough radiation can get out on the new frequency channels to balance the heat coming in, at which point the Earth's inputs and outputs balance, and the temperature holds steady there (for awhile - until we mess with the atmosphere's gases some more).
Why don't we just stop messing with the atmosphere's gases?